Liquid hydrogen – overview of a special energy carrier

Liquid hydrogen (LH₂) offers great potential for a sustainable and efficient hydrogen economy in Germany. However, the technology is still largely unknown. But what exactly is so interesting about this cryogenic liquid? We have summarized the most important facts for you.
Frequently asked questions about liquid hydrogen
What is the energy density of liquid hydrogen?
Liquid hydrogen has a high gravimetric (33.3 kWh/kg) and medium volumetric (2.4 kWh/l) energy density, making it significantly more space-efficient than gaseous hydrogen and lighter than ammonia or LOHCs (Liquid Organic Hydrogen Carriers) per amount of stored energy. These properties are particularly important for energy importing countries such as Germany, as liquid hydrogen can be transported and stored compactly.

At what temperature does hydrogen become liquid?
Hydrogen is usually gaseous. Hydrogen become liquid only at extremely low temperatures of around -253 degrees Celsius (20 degrees above absolute zero). The comparatively warm ambient air is sufficient for liquid hydrogen to become gaseous again. Energy can be saved if the liquid cools the surrounding components or processes as it warms up. This is practical, for example, for vehicles powered by fuel cells with LH2 in the tank.
 CC-Lizenz
CC-Lizenz
What is the connection between liquid hydrogen and superconductivity?
In superconducting DC cables, electricity can be transported extremely efficiently, without electrical losses and in a space-saving manner. The catch: the cable must be operated at low temperatures continuously (i.e. the ambient heat penetrating from outside must be removed). However, if high-temperature superconductors (HTS) are combined with liquid hydrogen, the cooling option is provided at virtually no extra cost. This synergy opens up completely new technological possibilities. One particularly exciting concept is the hybrid pipeline: Here, electricity flows in a superconducting cable in an LH₂ pipeline.
 Linde
Linde
Soon, liquid hydrogen tanks and pipelines could be cooling high-performance power transmission lines made of high-temperature superconductors.
What applications does liquid hydrogen have in practice?
Liquid hydrogen has been used as a rocket fuel for several decades. However, other vehicles with medium to large tanks also benefit from the high energy density of liquid hydrogen, such as trucks, freight trains, construction vehicles, ships and aircraft. They can achieve long ranges with comparatively small and light tanks and save on separate cooling systems.
In addition, regasified liquid hydrogen has an extremely high purity (99.9999 %), meaning that no extra purification processes are required. This is why it is already being used in semiconductor production and the chemical industry. There are also various applications for LH2 in steel and glass production.

This means it achieves significantly greater ranges than current electric trucks and lower tank volumes than diesel trucks –
and the optimization potential is far from exhausted.
Is liquid hydrogen safe?
Generally speaking, liquid hydrogen is no more or less dangerous than other fuels, it just behaves differently in many respects. Since liquid hydrogen is stored at low pressure, there is hardly any risk of a container rupturing, for example; however, any hydrogen that evaporates from LH2 tanks must be handled safely and sensibly.
Technical precautions for the safe handling of gaseous and liquid hydrogen are already common practice in industrial environments. Hydrogen technology can therefore achieve a good level of safety if handled appropriately.
Further information on safety considerations for hydrogen can be found at our partner institute ITES.
How does hydrogen become liquid?
To liquefy hydrogen, the gas is purified and then cooled down in several process steps: similar to a refrigerator, the gas is compressed, cooled and expanded again several times, cooling down a little further each time. When the gas is then expanded close to its boiling temperature of -253 °C, part of it condenses as liquid hydrogen and is collected in a separate container. The gaseous remainder is fed back into the cycle.
To date, a few dozen larger LH₂ liquefaction plants are in operation worldwide, but a significant increase in capacity is planned or currently under construction. As a rule of scale, the larger the plant, the more efficiently and cost-effectively hydrogen can be liquefied.

Storage and transportation of liquid hydrogen with almost no losses?
A tank for liquid hydrogen is usually spherical or cylindrical and consists of several walls with vacuum in between. During the storage period, a small proportion of the liquid hydrogen becomes gaseous, known as boil-off gas, and evaporation losses can also occur during refilling processes. However, suitable technology can minimize or even completely avoid these losses: For example, improved tank insulation and more efficient pumping systems are being discussed to ensure that less to no liquid hydrogen evaporates from storage tanks in the future. In addition, a rule of scale also applies here: losses decrease with increasing tank sizes – which is why studies are currently underway to produce even larger liquid hydrogen tanks. Boil-off gas produced in vehicle tanks can be used directly for propulsion.

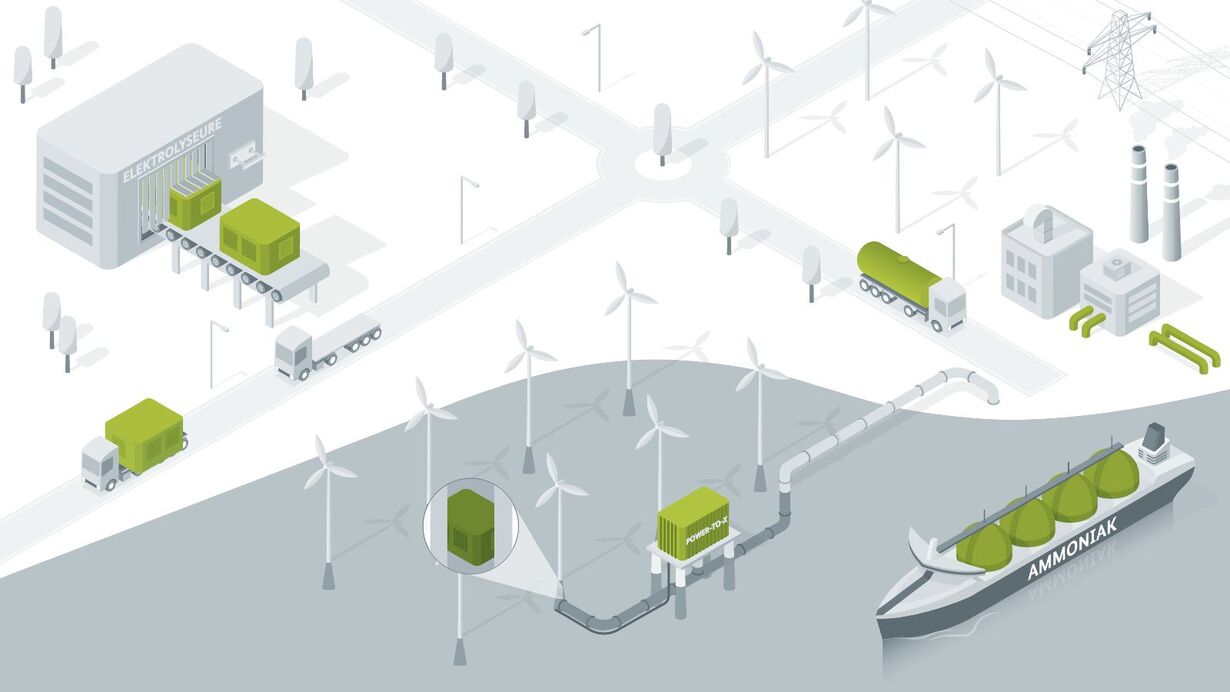
How can liquid hydrogen be imported to Germany?
To produce green, in other words climate-neutral hydrogen, you only need electricity and water (electrolysis). Electricity is also needed to liquefy the hydrogen. This is why liquid hydrogen is produced in places where a lot of cheap renewable energy is available, such as near offshore wind farms, large hydroelectric power plants or solar fields. From there, the liquid hydrogen can be transported to Germany by ship and distributed further by truck, train or pipeline. Ultimately, LH2 is used in a wide variety of applications, sometimes directly in liquid form and sometimes as a gas.

ITEP research on liquid hydrogen
Researchers at ITEP are coordinating the AppLHy! project, which is part of the TransHyDE flagship project of the German Hydrogen Strategy of the Federal Ministry of Education and Research. The focus of AppLHy! is on the provision, storage and transportation of liquid hydrogen (LH₂): One research focus at the ITEP is, for example, possible synergies of cryogenic liquid hydrogen with high-temperature superconducting components, drive systems and tapes. New functional and structural materials for use in liquid hydrogen applications are also being investigated and strategies for the safe use of the technologies are being developed.

Detailed insight into the current state of research on LH2
More information